Application Scenarios
High Resolution XPS Imaging
X-ray photoelectron spectroscopy (XPS), also known as chemical analysis electron spectroscopy (ESCA), is a surface sensitive analytical technique used to determine the elemental composition and chemical state of materials. It is based on the photoelectric effect, using X-rays to irradiate materials and excite electrons in the inner orbitals of atoms. By measuring the kinetic energy of these photoelectrons(EK)
EB=hν-ϕ-EK
Among them, h ν is the energy of the incident X-ray photon, and ϕ is the work function of the spectrometer. Binding energy is a characteristic of elements and their chemical environment, which can be used to identify the elements and their chemical states on the surface of materials. Due to its non-destructive, high surface sensitivity, and high element and chemical state recognition properties, XPS is widely used in fields such as materials science, nanotechnology, corrosion science, biology, and medicine.
USM-P By using spatial imaging technology, high spatial resolution (≤ 50 nm) XPS images can be obtained for high-resolution surface distribution analysis of elements or chemical states, making the analysis results more intuitive. Figure 1 shows an example of a partially oxidized gallium (110) crystal plane, with data obtained by scanning the Ge3d peak (Figure 1a). After data analysis, a chemical state distribution diagram (Figure 1b) and related photoelectron spectroscopy (Figure 1c) were obtained. A more intuitive understanding of the oxidation process and distribution。
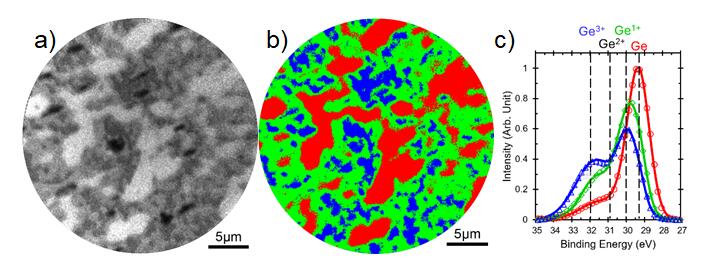
Figure 1:Chemical state image of partially oxidized gallium (110) crystal plane. a) XPS image (binding energy 29.3eV); b) Chemical state distribution diagram; c) Photoelectron spectra of various chemical states。
Video Presentation